The labelling of treated articles is regulated under the EU Biocidal Products Regulation (BPR, Regulation (EU) No 528/2012). Companies must comply with specific requirements when labelling treated articles. Proper and transparent labelling of such products is crucial for protecting consumer health, as well.
In addition to the EU BPR, the CLP and REACH Regulations must also be considered during the labelling process. While the CLP Regulation governs the classification and labelling of chemicals in products, the REACH Regulation controls the use of chemical substances. These regulations aim to ensure the accuracy and safety of the statements used on treated article labels.
Chemleg supports companies throughout the labelling process, helping them achieve regulatory compliance and contribute to chemical safety.
What Are the Labelling Obligations Under the EU BPR?
Labelling treated articles is one of the most effective ways for companies to demonstrate the safety of their products. Correct labelling helps prevent legal penalties and reputational damage.
Labelling is mandatory if:
- The treated article is claimed to have biocidal properties (e.g., “antibacterial” or “insect repellent”), and
- The active substance used in the biocidal product is considered hazardous to human health or the environment under Article 58(2) of the EU BPR.
The following decision diagram can be used to determine whether labelling of a treated article is required.
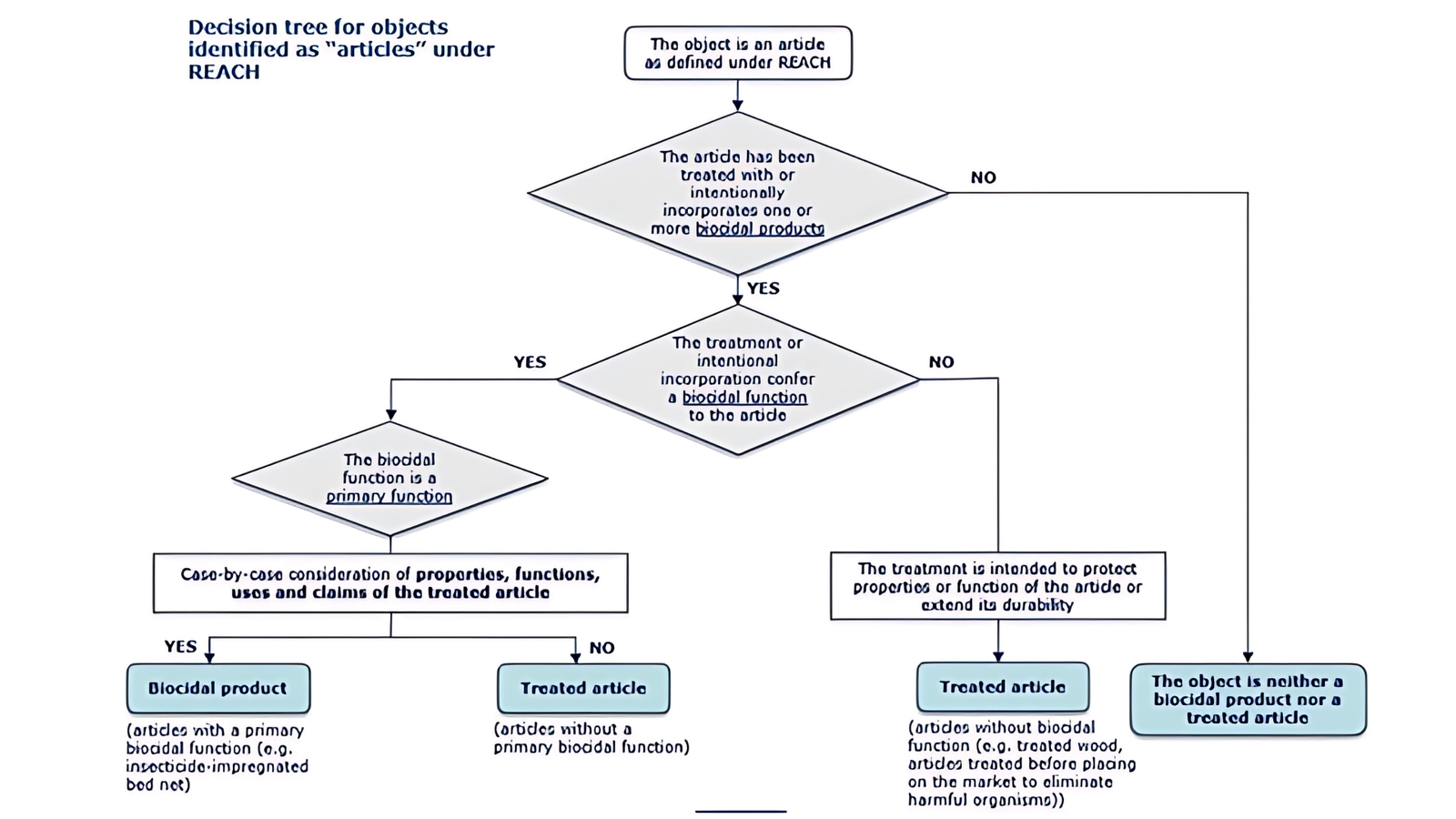
Information That Must Be Included on Labels of Treated Articles
The labels of treated articles must include the following information clearly and legibly:
|
Labelling Condition |
Mandatory? |
Required Information |
Explanation / Example |
|
If a biocidal product is used |
Yes |
Indicate that the treated article has been treated with a biocidal product. |
Example: “Contains a biocidal active substance.” |
|
If it contains active substances |
Yes |
Provide the name(s) of the active substance(s), with the IUPAC name if applicable. |
Example: “Contains: Silver Zinc Pyrithione” |
|
If biocidal claims are made |
Yes |
Any claimed biocidal properties must be stated on the label. |
Example: “Prevents bacterial growth” |
|
If consumer information is requested |
Yes |
Even if labelling is not mandatory, the supplier must provide the following information within 45 days upon request, free of charge: |
What Are the Steps of the Labelling Process?
To prepare labels in accordance with EU legislation, the following steps are followed:
- Identify the biocidal product used in the article.
- Determine the active substances involved.
- Review the claims made about the product.
- Draft the label text.
- Add required safety warnings.
- Ensure all information is clearly visible and legible on the label.
A sample label might look like this:
|
Product Name: Anti-bacterial Sports Socks Product Description: These socks are made with fabric treated with a biocidal product to help reduce the formation of odor-causing bacteria on the feet. Active Substance: Silver – IUPAC name: silver Biocidal Property: Helps prevent bacterial growth on fabric surfaces and reduces unpleasant odors. Instructions for Use: Wash in delicate cycle at up to 30°C. Information Notice: This is a treated article incorporating a biocidal active substance. Upon request, the name of the active substance and relevant safety information will be provided free of charge within 45 days. Warning: This product is not a medical device. The biocidal effect is limited to maintaining hygiene on the fabric surface. Manufacturer: X |
Reliable and Compliant Labelling Services with Chemleg
Chemleg provides professional support for labelling treated articles in compliance with the EU BPR. With its experienced team, Chemleg:
- Verifies the regulatory compliance of the biocidal products used,
- Analyzes product components,
- Prepares label texts in line with legal requirements,
- Manages communication with competent authorities when needed.
Contact us now for assistance.
Frequently Asked Questions
Which regulations must be considered for the labelling of treated articles?
The main legislation to be taken into account in the labelling of processed articles to be placed on the European Union market is the EU Biocidal Products Directive. In addition, compliance with the CLP and REACH Regulations and the Product Safety Directive 2001/95/EC is required.
Is labelling only required for consumer products?
No, treated articles intended for industrial use are also within the scope.
In which language should labels be prepared?
Labels must be written in the official language(s) of the country where the product is placed on the market.
Can labels be provided in digital form?
For physical products, the label must be affixed to the product. However, supplementary information may be provided via a QR code.
Is analysis mandatory for treated articles with biocidal properties?
Analysis is not mandatory. However, it may be necessary to demonstrate regulatory compliance, ensure quality control, meet customer expectations, and prevent potential issues.
What should be considered when choosing accredited laboratories for treated article analysis?
Laboratories should be accredited to ISO 17025 for the required analyses. Choose a laboratory that uses validated methods, has a strong quality management system, provides clear reporting, and has relevant experience.
What are the consequences for companies that fail to meet labelling obligations?
Companies that fail to fulfill their labeling obligations may face administrative fines, product withdrawal or recall from the market, sales bans or other penal sanctions. Such a situation will also significantly damage the company’s reputation and reliability.







